Situation at a Glance
Description of the Situation
Between 5 April (when the outbreak was initially detected) and 8 July 2022, 35 countries in five WHO Regions have reported 1010 probable cases (Figure 1) and 22 deaths. These include new and retrospectively identified cases since 1 October 2021, which fit the WHO case definition as stated below. There are three additional countries that have reported cases which are pending classification and are not included in the cumulative probable case count. Of the probable cases, 46 (5%) children have required transplants, and 22 (2%) deaths have been reported to WHO.
Almost half (48%) of the probable cases have been reported from the WHO European Region (21 countries reporting 484 cases), including 272 cases (27% of global cases) from the United Kingdom of Great Britain and Northern Ireland (the UK) (Table 1, Figure 2). The second highest number of probable cases have been reported from the Region of the Americas (n=435, including 334 cases (33% of global cases) from the United States of America), followed by the Western Pacific Region (n=70), the South-East Asia Region (n=19) and Eastern Mediterranean Region (n=2). Seventeen countries are reporting more than five probable cases. The actual number of cases may be underestimated, in part due to the limited enhanced surveillance systems in place. The case count is expected to change as more information and verified data become available.
Figure 1. Distribution of probable cases of severe acute hepatitis of unknown aetiology in children by country, as of 8 July 2022 (n=1010), 5 PM CEST

Table 1. Distribution of probable cases of severe acute hepatitis of unknown aetiology in children by WHO Region since 1 October 2021, as of 8 July 2022, 5 PM CEST
|
*The information included in this table contains data notified under IHR (2005), including from The European Surveillance System (TESSY) and official sources detected through event-based surveillance activities within the Public Health Intelligence process. Further information is presented in the Annex table.
⸸ Adenovirus positive in any specimen type (respiratory, urine, stool, whole blood, serum, other, or unknown specimen type)
Laboratory testing of cases
Based on the working case definition for probable cases (Box 1), laboratory testing has excluded hepatitis A-E viruses in these children. Pathogens like adenovirus and SARS-CoV-2 were detected by PCR in a number of the cases, although the data reported to WHO are incomplete.
Adenovirus continues to be the most frequently detected pathogen among cases with available data. In the European region, adenovirus was detected by PCR in 52% of cases (193/368) with available results (see Annex). In Japan, adenovirus was detected in 9% of cases (5/58) with known results. Due to limited adenovirus surveillance in most countries, it is challenging to assess whether these rates are higher than the expected rates in the population.
SARS-CoV-2 has been detected in a number of cases, however, data on serology results are limited. In the European region, SARS-CoV-2 was detected by PCR in 16% of cases (54/335) with available results (see Annex). Preliminary reports from the United States of America indicate that SARS-CoV-2 was detected in 8% of cases (15/197) with available results. In Japan, SARS-CoV-2 was also detected in 8% of cases (5/59) with available results. These figures may change as new data becomes available.
For further details, please refer to the EURO/ECDC Joint surveillance report, Japanese National Institute of Infectious Diseases report, UKHSA Case Update, UKHSA Third Technical Briefing, and the USCDC Technical Report.
Most reported cases did not appear to be epidemiologically linked; however, epidemiologically linked cases have been reported in Scotland, and the Netherlands.
Box 1. WHO Working case definition of severe acute hepatitis of unknown aetiology

Epidemiological characteristics of cases
Of 571 probable cases (57% of all probable cases) for which data are available, there has been a decreasing trend in cases over the last month (Figure 2). However, this trend should be interpreted carefully as there are reporting delays and limited surveillance in many countries.
Figure 2. Epidemiological curve of probable cases of severe acute hepatitis of unknown aetiology with available data, by week, by WHO region, as of 8 July 2022 (n=571), 5 PM CEST

Note: Figure 2 includes cases for which dates of symptom onset, hospitalization, or notification were reported to WHO (n= 571). The date of symptom onset was used when available (n=384). If unavailable, the week of hospitalization (n=163), or the week of notification (n=24), was used.
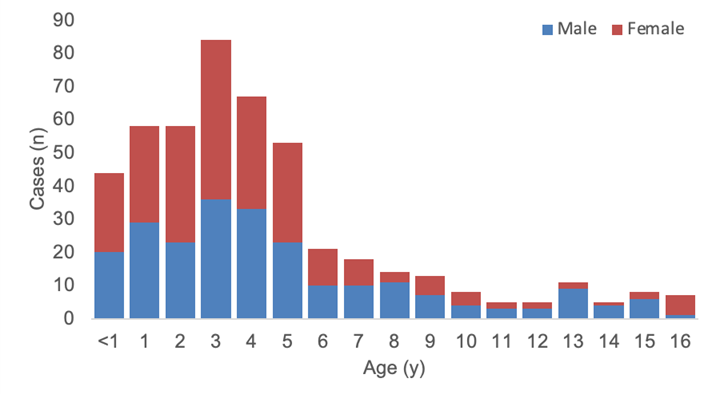
As of 8 July 2022, of 479 cases with information on gender and age, 48% are male (n=232), and the majority of cases (76%, n=364) are under six years of age (Figure 3).
Figure 3. Age and gender distribution of reported probable cases of severe acute hepatitis of unknown aetiology with available data, as of 8 July 2022 (n=479) 5 PM CEST
Out of 100 probable cases with available clinical data, the most commonly reported symptoms on presentation were nausea or vomiting (60% of cases), jaundice (53% of cases), general weakness (52% of cases) and abdominal pain (50% of cases).
Of all global cases with available data, a total of 167 cases (16% of all probable cases) had both date of symptom onset and date of hospitalization available. Among these, the median number of days between date of symptom onset and date of hospitalization was four days [interquartile range (IQR) 7].
Public Health Response
Epidemiological, clinical, laboratory, histopathological and toxicological investigations of the possible aetiology (or aetiologies) of the cases are underway by several national authorities, research networks, across different working groups in WHO and with partners. This includes detailed epidemiological investigations to identify common exposures, risk factors or links between cases.
On 11 July 2022, WHO launched a global online survey with an aim to estimate the incidence of severe acute hepatitis of unknown aetiology in 2022 compared to the previous five years, to understand where cases and liver transplants are occurring at higher-than-expected rates. WHO has shared the voluntary survey across nine global and regional networks of paediatric hepatologists and other specialist paediatricians working in major national units, requesting aggregated data as part of the global event investigation. Interim results of the survey will be made available publicly by WHO as soon as they are available.
The specific objectives of the survey are to:
- Assess whether there has been a recent increase or not in incidence of severe acute hepatitis of unknown aetiology (with and without acute liver failure) in children ≤16 years, including those requiring liver transplantation, in different countries and regions (number of cases in 2022 compared to 2017-2021)
- Assess any changes in the age distribution and severity of reported cases over time (2022 compared to 2017-2021).
WHO Risk Assessment
The risk at the global level is currently assessed as moderate considering the following factors:
- The aetiology of this severe acute hepatitis remains unknown and is being investigated.
- Limited epidemiological, laboratory, histopathological and clinical information are currently available to WHO.
- The actual number of cases and the geographical distribution may be underestimated, in part due to the limited enhanced surveillance systems in place.
- The possible mode of transmission of the aetiologic agent(s) has not been determined.
- Although there are still no available reports of healthcare-associated infections, human-to-human transmission cannot be ruled out following a few early reports of epidemiologically linked cases.
WHO Advice
Laboratory testing
WHO has developed interim guidance for Member States on testing considerations and strategies for suspect cases of severe acute hepatitis of unknown aetiology in children. The guidance includes advice to support Member States with diagnostic prioritization and can be modified for regional considerations of endemic diseases. The guidance also considers assessments for other aetiological factors of severe acute hepatitis in children, including other infectious agents, environmental exposures (toxins, medications), metabolic hereditary conditions, or autoimmune disorders, which should be considered in consultation with a paediatric hepatologist.
Prioritization should be given to routine collection of various specimens from as early after symptom onset as possible, to allow for later testing as required and to identify aetiology(ies). If laboratory capacity is limited, storage and referral to regional or global laboratories should be considered for the suggested investigative diagnostics. Any positive specimens should also be stored for further testing and/or investigation.
To further support Member States with laboratory testing, WHO is establishing a network of regional and global referral laboratories.
For more information, please see the Interim guidance on Laboratory testing for severe acute hepatitis of unknown aetiology in children.
Case reporting
WHO strongly encourages Member States to report cases of severe acute hepatitis of unknown aetiology in children matching WHO’s case definition, through established IHR mechanisms. For more information, please see the Suggested minimum variables for reporting cases of severe acute hepatitis of unknown aetiology in children.
Reporting clinical data through the WHO Global Clinical Platform
WHO has developed a clinical Case Report Form (CRF) to facilitate reporting of anonymized case-based data. The analysis of standardized global clinical data will contribute to understanding the aetiology as well as clinical characterization of disease, its natural history and severity; aiming to guide the public health response and the development of clinical management guidance including approaches to investigations and infection prevention and control interventions. WHO strongly encourages Member States’ participation in the Global Clinical Platform for all cases meeting the WHO case definition, even if the CRF cannot be fully completed. Patient clinical data may be collected prospectively or retrospectively through examination and review of medical records.
The clinical CRF can be accessed through the WHO Global Clinical Platform for severe acute hepatitis of unknown aetiology.
Infection Prevention and Control
Until more is known about the aetiology of these cases, WHO advises implementation of general infection prevention and control (IPC) practices including:
- Performing frequent hand hygiene, using soap and water or an alcohol-based hand-gel
- Avoiding crowded spaces and maintaining a distance from others
- Ensuring good ventilation when indoors
- Wearing a well-fitted mask covering your mouth and nose when appropriate
- Covering coughs and sneezes
- Using safe water for drinking
- Following the Five Keys to Safer Food: (1) keep clean; (2) separate raw and cooked; (3) cook thoroughly; (4) keep food at safe temperatures; and (5) use safe water and raw materials. Regular cleaning of frequently touched surfaces
- Staying home when unwell and seeking medical attention
Health facilities should adhere to standard precautions and implement contact and droplet precautions for suspected or probable cases.
Risk communication and community engagement
Until more is known about the aetiology of these cases and appropriate prevention measures, WHO advises that information is shared on general IPC practices. Efforts to communicate with empathy in a timely and transparent way, acknowledging what is known and unknown and what is being done to investigate will help to reassure parents and caregivers, maintaining trust in health authorities and interventions.
Annex table. Classification of reported probable cases of severe acute hepatitis of unknown aetiology by country since 1 October 2021, as of 8 July 2022
| WHO regions | Country | Probable/epi-linked cases* | Cases requiring liver transplants (cumulative 46) | SARS-CoV-2 positive by PCR (cumulative 78)# | Adenovirus positive by PCR (cumulative 209) ⸸ | Adenovirus type 41 (cumulative 31) |
|---|---|---|---|---|---|---|
| Europe | Austria | 3 | 0 | 1/3 | 0/3 | |
| Belgium | 14 | 0 | 3/14 | 2/7 | ||
| Bulgaria | 1 | 0 | 0 / 1 | 0/1 | ||
| Cyprus | 2 | 0 | 0 /1 | 1/2 | 0/1 | |
| Denmark | 8 | 0 | 2/8 | 0/7 | 0 | |
| France | 8 | 0 | 0 / 8 | 4/6 | 0/1 | |
| Greece | 12 | 0 | 0/9 | 2/10 | ||
| Ireland | 17 | 2 | 0 / 8 | 9/16 | ||
| Israel | 5 | 0 / 2 | 1 / 2 | |||
| Italy | 36 | 1 | 2/19 | 11/25 | ||
| Latvia | 1 | 0 | 1/1 | |||
| Luxembourg⸹ | 1 | 0 | 0/1 | 0/1 | ||
| Republic of Moldova | 1 | 0 | 0 / 1 | 0 / 1 | ||
| Netherlands | 15 | 3 | 1/4 | 4/9 | 0/0 | |
| Norway | 5 | 0 | 2/5 | 2/5 | 2/2 | |
| Poland | 11 | 0 | 0/2 | 2/5 | ||
| Portugal | 19 | 0 | 4/14 | 2/13 | 0/1 | |
| Serbia | 1 | 1 awaiting | 0 / 1 | 1/1 | ||
| Spain | 40 | 1 | 3/29 | 5/28 | 1/2 | |
| Sweden | 12 | 2, including 1 awaiting | 2/9 | 4/9 | ||
| United Kingdom (the) | 272 | 12 | 34/196 | 142/216 | 27 / 35 | |
| Americas | Argentina | 3 | 1 | 0 | 2 | 1 |
| Brazil | 2 | 0 | 0 | 0 | 0 | |
| Canada | 21 | 2 | 3/20 | 3/18 | 0/1 | |
| Colombia | 2 | 0 | 0 | 1 | 0 | |
| Costa Rica⸹ | 3 | 3 | ||||
| Mexico | 69 | 0 | ||||
| Panama | 1 | 0 | ||||
| United States of America | 334 | 21 | 15/197 | |||
| Western Pacific Region | Japan | 67 | 0 | 5/59 | 5/58 | 0 |
| Singapore | 3 | 0 | 1 | 1 | 0 | |
| Southeast-Asia | Indonesia | 18 | 0 | |||
| Maldives | 1 | 0 | ||||
| Eastern Mediterranean | occupied Palestinian territories | 1 | 0 | |||
| Qatar | 1 | 1 |
Blank cells indicate where no data was available at the time of this report.
*The information included in this table contains data notified under IHR (2005), including from The European Surveillance System (TESSY) and official sources detected through event-based surveillance activities within the Public Health Intelligence process.
#All specimens with known test result (negative, or positive) were included in the denominator.
⸸ Adenovirus positive in any specimen type (respiratory, urine, stool, whole blood, serum, other, or unknown specimen type) / number of cases with adenovirus test result in any specimen type. Any specimens with known test result (negative or positive) were included in the denominator.
⸹ Newly reported countries in this update
Further Information
- First report on acute hepatitis of unknown cause in Children in Japan, 30 June
- Joint ECDC-WHO Regional Office for Europe Hepatitis of Unknown Origin in Children Surveillance Bulletin, 1 July 2022
- WHO minimum reporting variables for severe acute hepatitis of unknown aetiology in children
- WHO interim guidance on laboratory testing for severe acute hepatitis of unknown aetiology
- WHO minimum reporting variables line list template
- WHO Global Clinical Platform
- WHO Disease Outbreak News; Multi-Country – Acute, severe hepatitis of unknown origin in children (24 June 2022)
- WHO Disease Outbreak News; Multi-Country – Acute, severe hepatitis of unknown origin in children (27 May 2022)
- The United Kingdom Health Security Agency (UKHSA) Acute Hepatitis Technical Briefing 3, (updated 19 May 2022)
- UKHSA case control study protocol (19 May 2022)
- Trends in Acute Hepatitis of Unspecified Etiology and Adenovirus Stool Testing Results in Children — United States, 2017–2022, Morbidity and Mortality Weekly Report (14 June 2022)
- United States Centers for Disease Control and Prevention Technical Report: Acute Hepatitis of Unknown Cause (22 June 2022)
Citable reference: World Health Organization (12 July 2022). Disease Outbreak News; Acute hepatitis of unknown aetiology in children - Multi-country. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON400
