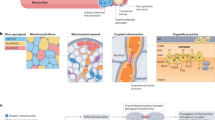
Abstract
To optimize its function, skeletal muscle exhibits exceptional plasticity and possesses the fundamental capacity to adapt its metabolic and contractile properties in response to various external stimuli (e.g., external loading, nutrient availability, and humoral factors). The adaptability of skeletal muscle, along with its relatively large mass and high metabolic rate, makes this tissue an important contributor to whole body health and mobility. This adaptational process includes changes in the number, size, and structural/functional properties of the myofibers. The adaptations of skeletal muscle to exercise are highly interrelated with dietary intake, particularly dietary protein, which has been shown to further potentiate exercise training-induced adaptations. Understanding the molecular adaptation of skeletal muscle to exercise and protein consumption is vital to elicit maximum benefit from exercise training to improve human performance and health. In this review, we will provide an overview of the molecular pathways regulating skeletal muscle adaptation to exercise and protein, and discuss the role of subsequent timing of nutrient intake following exercise.


Similar content being viewed by others
References
Fluck M (2006) Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J Exp Bio 209:2239–2248
Hood DA, Irrcher I, Ljubicic V, Joseph AM (2006) Coordination of metabolic plasticity in skeletal muscle. J Exp Bio 209:2265–2275
Fitts RH, Widrick JJ (1996) Muscle mechanics: adaptations with exercise-training. Exerc Sport Sci Rev 24:427–473
Yan Z, Okutsu M, Akhtar YN, Lira VA (2011) Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol 110:264–274
Hawley JA (2002) Adaptations of skeletal muscle to prolonged, intense endurance training. Clin Exp Pharmacol Physiol 29:218–222
Coffey VG, Hawley JA (2007) The molecular bases of training adaptation. Sport Med 37:737–763
Burd NA, Tang JE, Moore DR, Phillips SM (2009) Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol 106:1692–1701
Churchward-Venne TA, Burd NA, Phillips SM (2012) Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism. Nutr Metab 9:40
Tang JE, Phillips SM (2009) Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care 12:66–71
Moore, Camera DM, Areta JL, Hawley JA (2014) Beyond muscle hypertrophy: why dietary protein is important for endurance athletes. Appl Physiol Nutr Metab 39:1–11
Margolis LM, Pasiakos SM (2013) Optimizing intramuscular adaptations to aerobic exercise: effects of carbohydrate restriction and protein supplementation on mitochondrial biogenesis. Adv Nutr 4:657–664
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR (1997) Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 273:E99–E107
Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM (2011) Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 141:568–573
Joseph AM, Pilegaard H, Litvintsev A, Leick L, Hood DA (2006) Control of gene expression and mitochondrial biogenesis in the muscular adaptation to endurance exercise. Essays Biochem 42:13–29
Rose AJ, Richter EA (2009) Regulatory mechanisms of skeletal muscle protein turnover during exercise. J Appl Physiol 106:1702–1711
Rivas DA, Lessard SJ, Rice NP, Lustgarten MS, So K, Goodyear LJ, Parnell LD, Fielding RA (2014) Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J. doi:10.1096/fj.14-254490
Lessard SJ, Rivas DA, Stephenson EJ, Yaspelkis BB 3rd, Koch LG, Britton SL, Hawley JA (2011) Exercise training reverses impaired skeletal muscle metabolism induced by artificial selection for low aerobic capacity. Am J Physiol Regul Integr Comp Physiol 300:R175–R182
Rivas DA, Lessard SJ, Coffey VG (2009) mTOR function in skeletal muscle: a focal point for overnutrition and exercise. Appl Physiol Nutr Metab 34:807–816
Seyssel K, Alligier M, Meugnier E, Chanseaume E, Loizon E, Canto C, Disse E, Lambert-Porcheron S, Brozek J, Blond E, Rieusset J, Morio B, Laville M, Vidal H (2014) Regulation of energy metabolism and mitochondrial function in skeletal muscle during lipid overfeeding in healthy men. J Clin Endocrinol Metab 99:E1254–E1262
Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO (2002) Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16:1879–1886
Rivas DA, Lessard SJ, Saito M, Friedhuber AM, Koch LG, Britton SL, Yaspelkis BB 3rd, Hawley JA (2011) Low intrinsic running capacity is associated with reduced skeletal muscle substrate oxidation and lower mitochondrial content in white skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300:R835–R843
Hawley JA, Holloszy JO (2009) Exercise: it’s the real thing! Nutr Rev 67:172–178
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA (2003) PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol 284:C1669–C1677
Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Deriaz O (2003) Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52:2874–2881
Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I (2002) Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun 296:350–354
Terada S, Kawanaka K, Goto M, Shimokawa T, Tabata I (2005) Effects of high-intensity intermittent swimming on PGC-1alpha protein expression in rat skeletal muscle. Acta Physiol Scand 184:59–65
Terada S, Tabata I (2004) Effects of acute bouts of running and swimming exercise on PGC-1alpha protein expression in rat epitrochlearis and soleus muscle. Am J Physiol Endocrinol Metab 286:E208–E216
Bonen A (2009) PGC-1alpha-induced improvements in skeletal muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab 34:307–314
Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H (2008) PGC-1alpha is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab 294:E463–E474
Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM (2007) Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem 282:30014–30021
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797–801
Gurd BJ (2011) Deacetylation of PGC-1alpha by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab 36:589–597
Canto C, Auwerx J (2009) PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20:98–105
Jorgensen SB, Richter EA, Wojtaszewski JF (2006) Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J Physiol 574:17–31
Rivas DA, Yaspelkis BB 3rd, Hawley JA, Lessard SJ (2009) Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1β-D-ribofuranoside. J Endocrinol 202:441–451
Winder WW (2000) AMP-activated protein kinase: possible target for treatment of type 2 diabetes. Diabetes Technol Ther 2:441–448
Zheng D, MacLean PS, Pohnert SC, Knight JB, Olson AL, Winder WW, Dohm GL (2001) Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J Appl Physiol 91:1073–1083
Jager S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104:12017–12022
Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P (2007) Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26:1913–1923
Gurd BJ, Yoshida Y, McFarlan JT, Holloway GP, Moyes CD, Heigenhauser GJ, Spriet L, Bonen A (2011) Nuclear SIRT1 activity, but not protein content, regulates mitochondrial biogenesis in rat and human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 301:R67–R75
Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ (2010) A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588:1011–1022
Witczak CA, Jessen N, Warro DM, Toyoda T, Fujii N, Anderson ME, Hirshman MF, Goodyear LJ (2010) CaMKII regulates contraction- but not insulin-induced glucose uptake in mouse skeletal muscle. Am J physiol.Endocrinol Metab 298:E1150–E1160
Chin ER (2005) Role of Ca2 +/calmodulin-dependent kinases in skeletal muscle plasticity. J Appl Physiol 99:414–423
Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO (2007) Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol dChem 282:18793–18799
Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM (2003) An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci USA 100:7111–7116
Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z (2005) Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280:19587–19593
Garcia-Roves PM, Huss J, Holloszy JO (2006) Role of calcineurin in exercise-induced mitochondrial biogenesis. Am J physiology Endocrinol Metab 290:E1172–E1179
Lessard SJ, Rivas DA, Alves-Wagner AB, Hirshman MF, Gallagher IJ, Constantin-Teodosiu D, Atkins R, Greenhaff PL, Qi NR, Gustafsson T, Fielding RA, Timmons JA, Britton SL, Koch LG, Goodyear LJ (2013) Resistance to aerobic exercise training causes metabolic dysfunction and reveals novel exercise-regulated signaling networks. Diabetes 62:2717–2727
Puigserver P (2005) Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J obesity 29(Suppl 1):S5–S9
Richter EA, Kiens B, Wojtaszewski JF (2008) Can exercise mimetics substitute for exercise? Cell Metab 8:96–98
Saltin B, Henriksson J, Nygaard E, Andersen P, Jansson E (1977) Fiber types and metabolic potentials of skeletal muscles in sedentary man and endurance runners. Ann N Y Acad Sci 301:3–29
Phillips SM (2014) A brief review of critical processes in exercise-induced muscular hypertrophy. Sports med 44(Suppl 1):S71–S77
Carbone JW, McClung JP, Pasiakos SM (2012) Skeletal muscle responses to negative energy balance: effects of dietary protein. Adv Nutr 3:119–126
Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB (2009) Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 106:1374–1384
Proud CG (2007) Amino acids and mTOR signalling in anabolic function. Biochem Soc Trans 35:1187–1190
McClung JM, Lee WJ, Thompson RW, Lowe LL, Carson JA (2003) RhoA induction by functional overload and nandrolone decanoate administration in rat skeletal muscle. Pflugers Arch 447:345–355
McClung JM, Thompson RW, Lowe LL, Carson JA (2004) RhoA expression during recovery from skeletal muscle disuse. J Appl Physiol 96:1341–1348
Vissing K, Rahbek SK, Lamon S, Farup J, Stefanetti RJ, Wallace MA, Vendelbo MH, Russell A (2013) Effect of resistance exercise contraction mode and protein supplementation on members of the stars signalling pathway. J Physiol 591:3749–3763
Lamon S, Wallace MA, Leger B, Russell AP (2009) Regulation of stars and its downstream targets suggest a novel pathway involved in human skeletal muscle hypertrophy and atrophy. J Physiol 587:1795–1803
Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L (2005) The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem 41:173–186
Kandarian SC, Jackman RW (2006) Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33:155–165
Glickman MH, Maytal V (2002) Regulating the 26S proteasome. Curr Top Microbiol Immunol 268:43–72
Carbone JW, Margolis LM, McClung JP, Cao JJ, Murphy NE, Sauter ER, Combs GF Jr, Young AJ, Pasiakos SM (2013) Effects of energy deficit, dietary protein, and feeding on intracellular regulators of skeletal muscle proteolysis. FASEB J 27:5104–5111
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96:857–868
Hardie DG (2005) New roles for the LKB1– > AMPK pathway. Curr Opin Cell Biol 17:167–173
Winder WW, Hardie DG (1999) AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277:E1–E10
Wolfe RR, Miller SL (1999) Amino acid availability controls muscle protein metabolism. Diabetes Nutr Metab 12:322–328
Phillips SM, Hartman JW, Wilkinson SB (2005) Dietary protein to support anabolism with resistance exercise in young men. J Am Coll Nutr 24:134S–139S
Farup J, Rahbek SK, Vendelbo MH, Matzon A, Hindhede J, Bejder A, Ringgard S, Vissing K (2013) Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand J Med Sci Sports. doi:10.1111/sms.12083
Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM (2007) Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 86:373–381
Wolfe RR (2002) Regulation of muscle protein by amino acids. J Nutr 132:3219S–3224S
Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR (2000) An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 88:386–392
Pasiakos SM (2012) Exercise and amino acid anabolic cell signaling and the regulation of skeletal muscle mass. Nutrients 4:740–758
Tipton KD, Gurkin BE, Matin S, Wolfe RR (1999) Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem 10:89–95
Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D (2009) A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 109:1582–1586
Robinson MJ, Burd NA, Breen L, Rerecich T, Yang Y, Hector AJ, Baker SK, Phillips SM (2013) Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl Physiol Nutr Metab 38:120–125
Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ (2005) Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19:422–424
Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM (2009) Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89:161–168
Bohe J, Low A, Wolfe RR, Rennie MJ (2003) Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol 552:315–324
Phillips SM (2009) Physiologic and molecular bases of muscle hypertrophy and atrophy: impact of resistance exercise on human skeletal muscle (protein and exercise dose effects). Appl Physiol Nutr Metab 34:403–410
Deutz NE, Wolfe RR (2013) Is there a maximal anabolic response to protein intake with a meal? Clin Nutr 32:309–313
Borgenvik M, Apro W, Blomstrand E (2012) Intake of branched-chain amino acids influences the levels of MAFbx mRNA and MuRF-1 total protein in resting and exercising human muscle. Am J Physiol Endocrinol Metab 302:E510–E521
Reitelseder S, Agergaard J, Doessing S, Helmark IC, Schjerling P, van Hall G, Kjaer M, Holm L (2014) Positive muscle protein net balance and differential regulation of atrogene expression after resistance exercise and milk protein supplementation. Eur J Nutr 53:321–333
Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, Rasmussen BB (2010) Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol 299:R533–R540
Stefanetti RJ, Lamon S, Rahbek SK, Farup J, Zacharewicz E, Wallace MA, Vendelbo MH, Russell AP, Vissing K (2014) Influence of divergent exercise contraction mode and whey protein supplementation on atrogin-1, MuRF1, and FOXO1/3A in human skeletal muscle. J Appl Physiol 116:1491–1502
Camera DM, West DW, Phillips SM, Rerecich T, Stellingwerff T, Hawley JA, Coffey VG (2014) Protein Ingestion Increases Myofibrillar Protein Synthesis after Concurrent Exercise. Med Sci Sports Exerc
Sun X, Zemel MB (2009) Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr Metab 6:26
Bruckbauer A, Zemel MB (2014) Synergistic effects of polyphenols and methylxanthines with leucine on AMPK/Sirtuin-mediated metabolism in muscle cells and adipocytes. PLoS ONE 9:e89166
Bruckbauer A, Zemel MB (2011) Effects of dairy consumption on SIRT1 and mitochondrial biogenesis in adipocytes and muscle cells. Nutr Metab 8:91
Howarth KR, Moreau NA, Phillips SM, Gibala MJ (2009) Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. J Appl Physiol 106:1394–1402
Pasiakos SM, McClung HL, McClung JP, Margolis LM, Andersen NE, Cloutier GJ, Pikosky MA, Rood JC, Fielding RA, Young AJ (2011) Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr 94:809–818
Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM (2009) Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol 587:897–904
Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ (2008) Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586:3701–3717
Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, Phillips SM, Hawley JA (2011) Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur J Appl Physiol 111:1473–1483
Breen L, Phillips SM (2011) Skeletal muscle protein metabolism in the elderly: interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab 8:68
Taylor C, Bartlett JD, van de Graaf CS, Louhelainen J, Coyne V, Iqbal Z, Maclaren DP, Gregson W, Close GL, Morton JP (2013) Protein ingestion does not impair exercise-induced AMPK signalling when in a glycogen-depleted state: implications for train-low compete-high. Eur J Appl Physiol 113:1457–1468
Hill KM, Stathis CG, Grinfeld E, Hayes A, McAinch AJ (2013) Co-ingestion of carbohydrate and whey protein isolates enhance PGC-1alpha mRNA expression: a randomised, single blind, cross over study. J Int Soc Sports Nutr 10:8
Schoenfeld BJ, Aragon AA, Krieger JW (2013) The effect of protein timing on muscle strength and hypertrophy: a meta-analysis. J Int Soc Sports Nutr 10:53
Mitchell CJ, Churchward-Venne TA, Parise G, Bellamy L, Baker SK, Smith K, Atherton PJ, Phillips SM (2014) Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS ONE 9:e89431
Service. UAR (2012) Energy intakes: percentages of energy from protein, carbohydrate, fat, and alcohol, by gender and age, what we eat in America, NHANES 2009-2010. Avialable from:http://www.ars.usda.gov/ba/bhnrc/fsrg
West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM (2011) Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr 94:795–803
Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, Phillips SM (2014) Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 99:276–286
Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, Hawley JA, Coffey VG (2013) Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 591:2319–2331
Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, Layman DK, Paddon-Jones D (2014) Dietary Protein Distribution Positively Influences 24-h Muscle Protein Synthesis in Healthy Adults. J Nutr 144:876–880
Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ (2010) Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92:1080–1088
Bohe J, Low JF, Wolfe RR, Rennie MJ (2001) Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 532:575–579
Millward DJ, Pacy PJ (1995) Postprandial protein utilization and protein quality assessment in man. Clin Sci 88:597–606
Souba WW, Pan M, Stevens BR (1992) Kinetics of the sodium-dependent glutamine transporter in human intestinal cell confluent monolayers. Biochem Biophys Res Commun 188:746–753
Palacin M, Estevez R, Bertran J, Zorzano A (1998) Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev 78:969–1054
Nishimura M, Naito S (2008) Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab Pharmacokinet 23:22–44
Baird FE, Bett KJ, MacLean C, Tee AR, Hundal HS, Taylor PM (2009) Tertiary active transport of amino acids reconstituted by coexpression of system A and L transporters in xenopus oocytes. Am J Physiol Endocrinol Metab 297:E822–E829
Peyrollier K, Hajduch E, Blair AS, Hyde R, Hundal HS (2000) l-leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the l-leucine-induced up-regulation of system a amino acid transport. Biochem J 350(Pt 2):361–368
Liu XM, Reyna SV, Ensenat D, Peyton KJ, Wang H, Schafer AI, Durante W (2004) Platelet-derived growth factor stimulates LAT1 gene expression in vascular smooth muscle: role in cell growth. FASEB J 18:768–770
Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB (2010) An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 298:E1011–E1018
Jewell JL, Russell RC, Guan KL (2013) Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol 14:133–139
Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM (2008) The rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320:1496–1501
Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM (2010) Ragulator-rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141:290–303
Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL (2008) Regulation of TORC1 by rag GTPases in nutrient response. Nature Cell Biol 10:935–945
Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM (2012) Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150:1196–1208
Acknowledgments
This material is based upon the work supported by the U.S. Department of Agriculture (USDA), under agreement No. 58-1950-0014. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. DAR is supported by a RCDC fellowship from the Boston Claude D. Pepper Center OAIC (1P30AG031679).
Conflict of interest
L. M. Margolis and D. A. Rivas have nothing to disclose.
Human and Animal Rights and Informed Consent
To the best of author’s knowledge, all procedures performed in the cited studies involving human participants in this review were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Margolis, L.M., Rivas, D.A. Implications of Exercise Training and Distribution of Protein Intake on Molecular Processes Regulating Skeletal Muscle Plasticity. Calcif Tissue Int 96, 211–221 (2015). https://doi.org/10.1007/s00223-014-9921-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9921-0